Unraveling Xylomannan – natural beetle antifreeze compound
 Animals and plants living in snow and sub-zero areas have evolved all sorts of chemical tricks that allow them to live in these cold environments. There are various natural antifreeze compounds from such organisms, but researchers from RIKEN Advanced Science Institute at Wako focused on one called xylomannan – a natural antifreeze produced by the freeze-tolerant Alaskan beetle Upis ceramboides.
Animals and plants living in snow and sub-zero areas have evolved all sorts of chemical tricks that allow them to live in these cold environments. There are various natural antifreeze compounds from such organisms, but researchers from RIKEN Advanced Science Institute at Wako focused on one called xylomannan – a natural antifreeze produced by the freeze-tolerant Alaskan beetle Upis ceramboides.
Upis ceramboides is a wood-living species of beetle which lives in quantities below the bark of fire-damaged birches. Although it used to be common in southern Sweden, now it resides in the Norrland coast (Västerbotten and Norrbotten) as well as Canada and Alaska. In order to survive harsh conditions during cold periods of the year, the species developed xylomannan – a non-protein antifreeze molecule (sugar (saccharide) and a fatty acid) as well as the sugar threitol which enable it to prolong its existence long enough to reproduce themsleves.
Xylomannan was first reported in 2009, and has been shown to be amongst the most active insect antifreezes found to date. Antifreeze compounds, which are also known as thermal hysteresis factors (THFs), protect the insects’ cells from damage as temperatures fall and ice crystals begin to form. THFs seem to work by sticking to the surface of ice crystals that begin to form and stopping them from further development, thus protecting nearby cell membranes from damage caused by crystals.
“Xylomannan is the first example of a THF biomolecule with little or no protein component”, said Akihiro Ishiwata, from the RIKEN Advanced Science Institute at Wako. “Its mode of action is not entirely clear, but it should be different to those of common THFs such as antifreeze proteins and glycoproteins.”
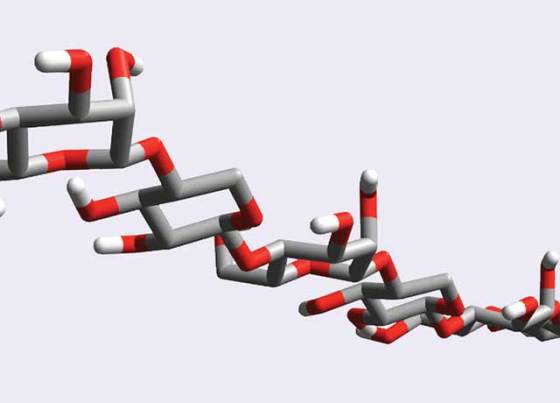
What makes it special is the fact that unlike other natural THFs isolated to date which are protein based, xylomannan is consisted from a long-chain sugar-based compound named glycan.
In order to confirm the proposed structure of xylomannan, Ishiwata and his colleague Yukishige Ito studied how the material interacts with ice crystals. They synthesized what they thought to be a key component of the compound’s sugar-based backbone, and used nuclear magnetic resonance techniques and molecular modeling for structural analysis.
The results not only confirmed their assumption that the structure matches that of the natural compound, but it also revealed that xylomannan has the potential to be hydrophilic on the one of its sides, while being hydrophobic on its other side. The hydrophobic surface should repel water molecules away from the ice crystal, stopping it from growing any further.
“We propose that the hydrophilic phase of xylomannan might bind to the ice crystal, exposing the hydrophobic phase on the ice crystal’s surface”, said Ishiwata. “However, the binding mode is still not clear from our structural analysis.”
To test the theory further, the team now plans to synthesize longer fragments of xylomannan to examine their ice-binding ability. Although xylomannan has intrigued researchers due to its property that allows these beetles to cryopreserve their tissues at relatively low concentrations, if the researchers prove their claims, it could find variable new uses where sides of a single membrane have hydrophilic and hydrophobic properties.
For more information, read the article published in the Journal of the American Chemical Society: “Synthetic study and structural analysis of the antifreeze agent xylomannan from Upis ceramboides”.









Leave your response!