Egg-yolk inspired sulfure cathodes set record battery capacity
 Researchers from the SLAC National Accelerator Laboratory, which is operated by the Stanford University, managed to set a world record for energy storage in the sulfur cathode of a rechargeable lithium-ion battery. They came up with a novel “yolk-shell” design which enables these batteries to store five times more energy compared to current commercially available batteries. The technology could lead to new generations of lighter, longer-lasting batteries for use in portable electronics and electric vehicles.
Researchers from the SLAC National Accelerator Laboratory, which is operated by the Stanford University, managed to set a world record for energy storage in the sulfur cathode of a rechargeable lithium-ion battery. They came up with a novel “yolk-shell” design which enables these batteries to store five times more energy compared to current commercially available batteries. The technology could lead to new generations of lighter, longer-lasting batteries for use in portable electronics and electric vehicles.
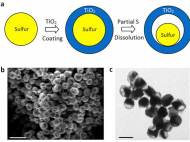
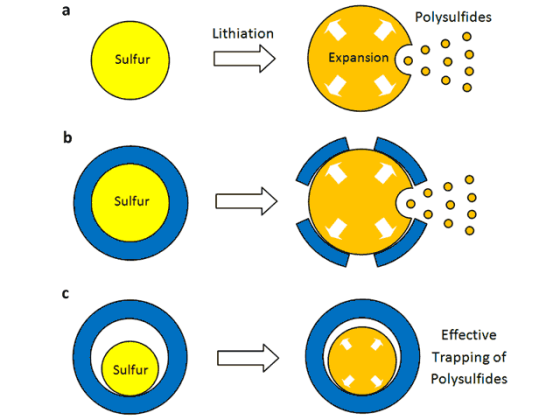
Lithium-ion batteries work by moving lithium ions back and forth between two electrodes, the cathode and anode. Although researchers have presumed that sulfur could theoretically store more lithium ions and thus much more energy for nearly 2 decades, two critical disadvantages prevented its commercial use. When lithium ions enter a sulfur cathode during the discharge process, they bond with sulfur atoms to create an intermediate compound that’s important for the cathode’s performance. However, this compound keeps on dissolving, thus limiting the cathode’s energy-storage capacity. Although researchers tried to develop a protective coating which prevents the intermediate compound from dissolving, the influx of ions caused the cathode to expand and crack the coating.
Led by Yi Cui, a Stanford associate professor of materials science and engineering and a member of the Stanford Institute for Materials and Energy Sciences, SLAC researchers came up with a cathode made of nanoparticles, each a tiny sulfur nugget surrounded by a hard shell of porous titanium dioxide, like an egg yolk in an eggshell. Each cathode particle is only 800nm in diameter, about one-hundredth the diameter of a human hair. Between the yolk and shell, where the egg white would be, is an empty space into which the sulfur can expand.
During discharging, lithium ions pass through the shell and bind to the sulfur, which expands to fill the without breaking the shell. The shell, meanwhile, protects the sulfur-lithium intermediate compound from electrolyte solvent that would dissolve it.
“After 1,000 charge/discharge cycles, our yolk-shell sulfur cathode had retained about 70 percent of its energy-storage capacity. This is the highest performing sulfur cathode in the world, as far as we know”, said Cui. “Even without optimizing the design, this cathode cycle life is already on par with commercial performance. This is a very important achievement for the future of rechargeable batteries.”
SLAC researchers plan to apply a similar approach to develop a yolk-shell silicon anode in order to produce a long-lasting battery with high-energy.
For more information, read the article published in the Nature Communications: “Sulphur–TiO2 yolk–shell nanoarchitecture with internal void space for long-cycle lithium–sulphur batteries”.









Leave your response!