Using bacteria mechanism to create bonds
 Researchers from the University of Oxford managed to create a bio-inspired superglue with unmatched performance by engineering an unusual protein from Streptococcus pyogenes – a type of bacteria that can cause mild infections as well as some life-threatening diseases. The modified protein enables sticking molecules, proteins or enzymes together to build new structures on the nanometer scale.
Researchers from the University of Oxford managed to create a bio-inspired superglue with unmatched performance by engineering an unusual protein from Streptococcus pyogenes – a type of bacteria that can cause mild infections as well as some life-threatening diseases. The modified protein enables sticking molecules, proteins or enzymes together to build new structures on the nanometer scale.
“The protein is special because it naturally reacts with itself and forms a lock”, said Dr Mark Howarth, who developed the superglue with his graduate student Bijan Zakeri at the Department of Biochemistry.
All proteins consist of amino acids that are linked together into long chains by strong covalent bonds. Inspired by the ability of S. pyogenes to use an extra covalent bond of FbaB protein to attach and invade human cells, the researchers developed superglue capable to form nearly instant binds with exceptional strength.
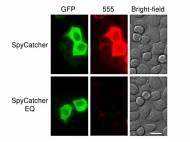
The researchers managed to use this ability by splitting the protein around this extra covalent bond. They’ve nicknamed the larger fragment which formed the bulk of the original protein SpyCatcher. Once SpyCatcher gets hold of the shorter protein segment, dubbed SpyTag, they bond within minutes with high yield.
Mark claims that there isn’t really any equivalent way to bind biomolecules together, since there are other methods that require chemical reactions to join two proteins together covalently but often only small proportions react, they take a long time, or they require UV light, toxic catalysts or reaction conditions that could damage living cells.
In order to test the strength of the bond, the researchers collaborated with researchers from the University of Miami who used atomic force microscope to measure the force needed to pull apart SpyTag from SpyCatcher. But when they pulled on each end, the chemical links holding the proteins to the apparatus broke first.
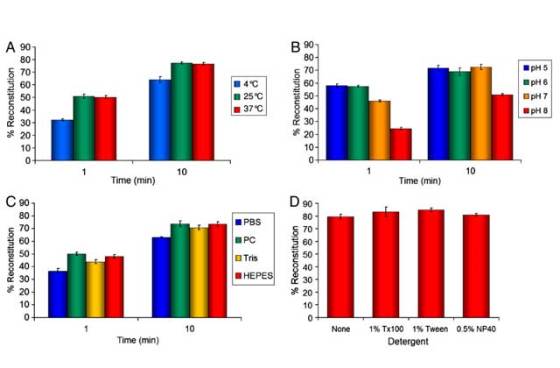
The researchers claim that it doesn’t matter whether the linking occurs in acidic or neutral conditions, or whether it is 4°C or 37°C (37°F or 98°F) and, once bonded, even boiling in detergent won’t separate the protein fragments. They also claim it acts as Velcro, since the parts stick to each other without sticking to other things.
Mark and his team are now working on developing the molecular superglue technology through Isis Innovation, the University of Oxford’s technology transfer company. The ability to attach SpyCatcher and SpyTag onto other molecules could be used in research labs in order to hold structures within biological cells and resist the forces generated by important motors, machines and transporters inside the cell. It could be used in creation of systems that mimic photosynthesis with desired waste products, or to connect all the enzymes involved in a chemical process in order to speed up reactions and increase yields.
For more information, you can read the paper published in the PNAS: “Peptide tag forming a rapid covalent bond to a protein, through engineering a bacterial adhesion”.









This technology could induce a large variety of new breakthroughs!
My congratulations to people involved.